
Clinical Research
1. Heart Disease Genetics
Learn more by calling (480) 728-9979 (East Valley) | (602) 406-1156 (Phoenix/Downtown)
Can genetics predict risk for Heart Disease?
Participate in a new research study to learn if you are at risk for heart disease.
What is the purpose of the study?
The purpose of this free study is to confirm the genetic risk score as a screening test for prevention of coronary artery disease, as this is the cause of chest pain, heart attacks and cardiac death.
What is genetic testing?
Identifying genetic risks for cardiovascular diseases may lead to personalized treatment and better outcomes. Because inherited heart conditions can run in families, your results can help your family members identify underlying conditions for proactive care.
Who can take part?
- Males or females 30-60 years of age
- No known coronary artery disease
- Willing and able to sign an Informed Consent Form
Why do testing?
- Coronary Artery Disease is an epidemic—the most common cause of death in the world.
- An American has a 50% chance of experiencing a cardiac event during a normal lifespan.
- Genetic risk for CAD is preventable.
There is no cost to participate.
Heart Disease Genetic Study Brochure
Heart Disease Genetic Study Poster
2. Triluminate Trial
To learn more, please call (602) 842-7352.
St. Joseph’s Hospital and Medical Center is pleased to participate in the Triluminate Research trial. The TRILUMINATE Pivotal Trial is studying the TriClip™ Tricuspid Valve Repair System in patients with symptomatic severe tricuspid regurgitation who are deemed appropriate for transcatheter intervention. Approximately 700 subjects will be studied at up to 80 medical centers in Europe, United States and Canada. A qualified team of doctors will monitor the patients included in the TRILUMINATE Pivotal Trial. Study participants will play an important role in helping doctors evaluate transcatheter tricuspid valve repair as an option for patients with tricuspid regurgitation.
 WHAT IS TRICUSPID VALVE REPAIR?
WHAT IS TRICUSPID VALVE REPAIR?
Transcatheter tricuspid valve repair is an investigational treatment for patients who are deemed appropriate for this procedure. The procedure is done using the Abbott TriClip™ repair system in a minimally invasive manner without the need to temporarily stop the heart.
- An implantable clip (A) is delivered into the heart by a catheter, or tube (B), via an incision in the upper leg.
- Once the clip is put into place on the tricuspid valve, the catheter is removed.
- The clip becomes a permanent implant in the heart, allowing the valve to close tightly and restore normal blood flow through the heart.
WHAT IS INVOLVED IF I CHOOSE TO PARTICIPATE?
Before you are enrolled, you will be screened to make sure you are a good candidate for the study.
Your screening may include:
- An in-person visit with a heart failure specialist
- An echocardiogram (ECHO) to determine if you have the appropriate valve anatomy
- A review of your medical history
- Blood tests
You can still see your personal cardiologist for regular checkups and care. In addition, you will be monitored by regular follow-up exams.
WHAT HAPPENS IF I PASS THE SCREENING PROCESS?
- You will be assessed in-person at the treatment center and then registered in the study.
- Your treatment group will be identified.*
- You will begin treatment.
- You will also have follow-up exams at the following intervals:
- 30 days
- 6 months
- 1 year
- 18 months
- 2 years
- 3 years
- 4 years
- 5 years
CAUTION: Investigational device. Limited by federal (U.S.) law to investigational use only.
WHO CAN PARTICIPATE IN THE TRILUMINATE PIVOTAL TRIAL?
Both men and women can participate.
You may qualify if:
- You have been diagnosed with symptomatic severe tricuspid regurgitation
- It has been determined by the study site's heart team that transcatheter intervention is appropriate for you
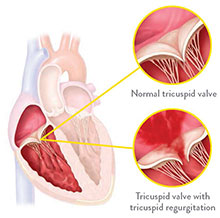
WHAT IS TRICUSPID REGURGITATION?
Tricuspid regurgitation is a condition in which the heart's tricuspid valve doesn't close properly. When this happens, some blood flows backward through the valve. The heart must then work harder to push blood through the body, which can cause fatigue, shortness of breath, and worsening heart failure. Tricuspid regurgitation is caused by abnormalities of the surrounding valve structures most commonly due to heart disease. These abnormalities cause the tricuspid valve to enlarge and thus allow blood to flow in a backwards direction.
HOW IS TRICUSPID REGURGITATION TREATED?
There are two main treatments for tricuspid regurgitation: MEDICAL THERAPY and TRICUSPID VALVE SURGERY. Prescription medicine can be taken to prevent symptoms associated with tricuspid regurgitation. Medicines include beta-blockers, angiotensin-converting enzyme (ACE) inhibitors, vasodilators, and diuretics. Surgery to repair or replace the tricuspid valve may be an option for patients with tricuspid regurgitation. While surgery is an effective treatment for some cases of tricuspid regurgitation, it is a major procedure with associated risks. Patients who are elderly, have advanced heart failure, or have other serious medical conditions may not be appropriate for surgery.
WHAT IS INVOLVED IF I CHOOSE TO PARTICIPATE?
Before you are enrolled, you will be screened to make sure you are a good candidate for the study.
Your screening may include:
- An in-person visit with a heart failure specialist
- An echocardiogram (ECHO) to determine if you have the appropriate valve anatomy
- A review of your medical history
- Blood tests

To learn more, please call (602) 842-7352.
